ABOUT IPSEN
Our team of over 5,000 talented and dedicated professionals around the world works together every day to innovate for the benefit of patients and caregivers.

Making an impact
Our purpose-led people are passionate about making a real impact on patients’ lives every day. We do this by striving to discover, innovate and bring to market pharmaceutical products that will make a difference in the lives of patients, their caregivers and healthcare professionals.

Leading the way
With a focus on transformative medicines in oncology, rare disease and neuroscience, our vision is to be a leading global mid-size biopharmaceutical company. With the right combination of size and agility, we continue to push ourselves as a business and as individuals to deliver positive change.
Our strategy
Our strategy, Focus. Together. For patients & society, consists of four strategic priorities that guide our decision-making.
We are focused on ensuring patients have access to our treatments, collaborating with them and with patient organizations to unlock key insights that will allow us to deliver advancements. We work tirelessly to ensure patients have access to our innovative treatments.
We’re committed to building a robust, sustainable pipeline across all stages of development and in each of our three therapy areas so that we can continue to deliver innovative treatments, now and in the future. We seek out biotechs, academic institutions and other partners who share our vision of making a real impact for patients, and we aim to deliver at least one molecular entity or meaningful indication every year.
We focus our efforts and resources where they will move the needle for patients. By creating efficiencies, we can make the right investments at the right time to bring new, innovative therapies to patients around the world.
Our team of over 5,000 talented and dedicated professionals around the world endeavors every day to develop and build our capabilities. With our science-led and patient-driven approach, we grow together in a supportive environment.

Our ESG commitment
Our mission is clear: to prolong and improve patients’ lives and health outcomes and make a positive impact for society. Our ESG framework guides us as we deliver positive change across four key areas: Environment, Patients, People and Governance.

It’s an exciting time to be at Ipsen and I’m grateful to our dedicated and passionate teams worldwide. Our acceleration in our pipeline and our growth shows that we are on the right track and making a real impact for patients.
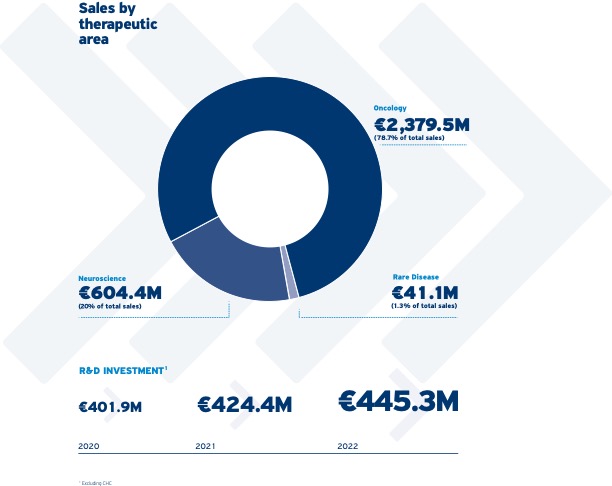
2022: a year of accelerated momentum and growth
Bolstered by our company strategy, we accelerated our strong momentum and saw record sales in 2022. Our growth platforms, focused on our three therapeutic areas, delivered double-digit sales growth.
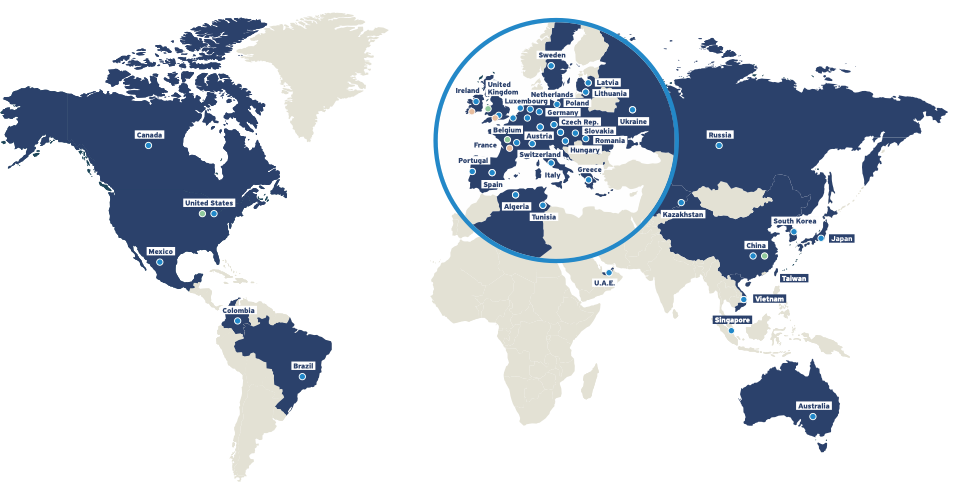
Our global presence
Ipsen’s products are registered in 88 countries. With 5,000 employees working to create value for patients and society around the world, we have global reach while remaining an agile, mid-sized company.
FONDATION IPSEN
Rare but not alone
Functioning independently of Ipsen, Fondation Ipsen is dedicated to increasing scientific knowledge and patient research. Focused on improving the lives of patients with rare diseases, Fondation Ipsen improves understanding of rare diseases through information-sharing and strategic partnerships with key actors in the rare disease community.
Our history
Founded as a family firm, Ipsen has been innovating for patients for nearly 100 years. And we aim to keep growing and innovating for our patients for at least 100 more.
The Ipsen story begins
Dr. Henri Beaufour founded the Beaufour Laboratories in Dreux. The first product marketed was Romarene®, a rosemary-based medicine intended for the treatment of digestive disorders.
Expansion: new factories & centers
Laboratoires Beaufour underwent a phase of expansion. In 1954, the group launched betaine citrate, used in the symptomatic treatment of dyspepsia. Henri Beaufour’s two sons, Albert and Gérard Beaufour, joined the company.
Expansion: new factories & centers
The group opened a factory in Dreux in 1961, and another in L’Isle-sur-la-Sorgue in 1965. A research center opened in Plessis-Robinson the same year.
The Ipsen brand is launched
Laboratoires Beaufour created a subsidiary, Ipsen, in 1975, and began to internationalize its activities. In 1976, the company opened a research center in Hopkinton (Massachusetts) in the U.S. In 1977, the group launched Smecta®, a diosmectite clay used as a gastrointestinal bandage and anti-diarrheal agent.
Fondation Ipsen created, and Decapeptyl brought to market
In 1983, the group created the Fondation Ipsen under the aegis of the Fondation de France, to encourage exchanges between scientists in the field of life sciences. In 1986, the group launched Decapeptyl®, used to treat certain pathologies influenced by sex hormones, such as prostate cancer, endometriosis, uterine fibroids and early puberty.
